Answers
Answer:
See answer below
Explanation:
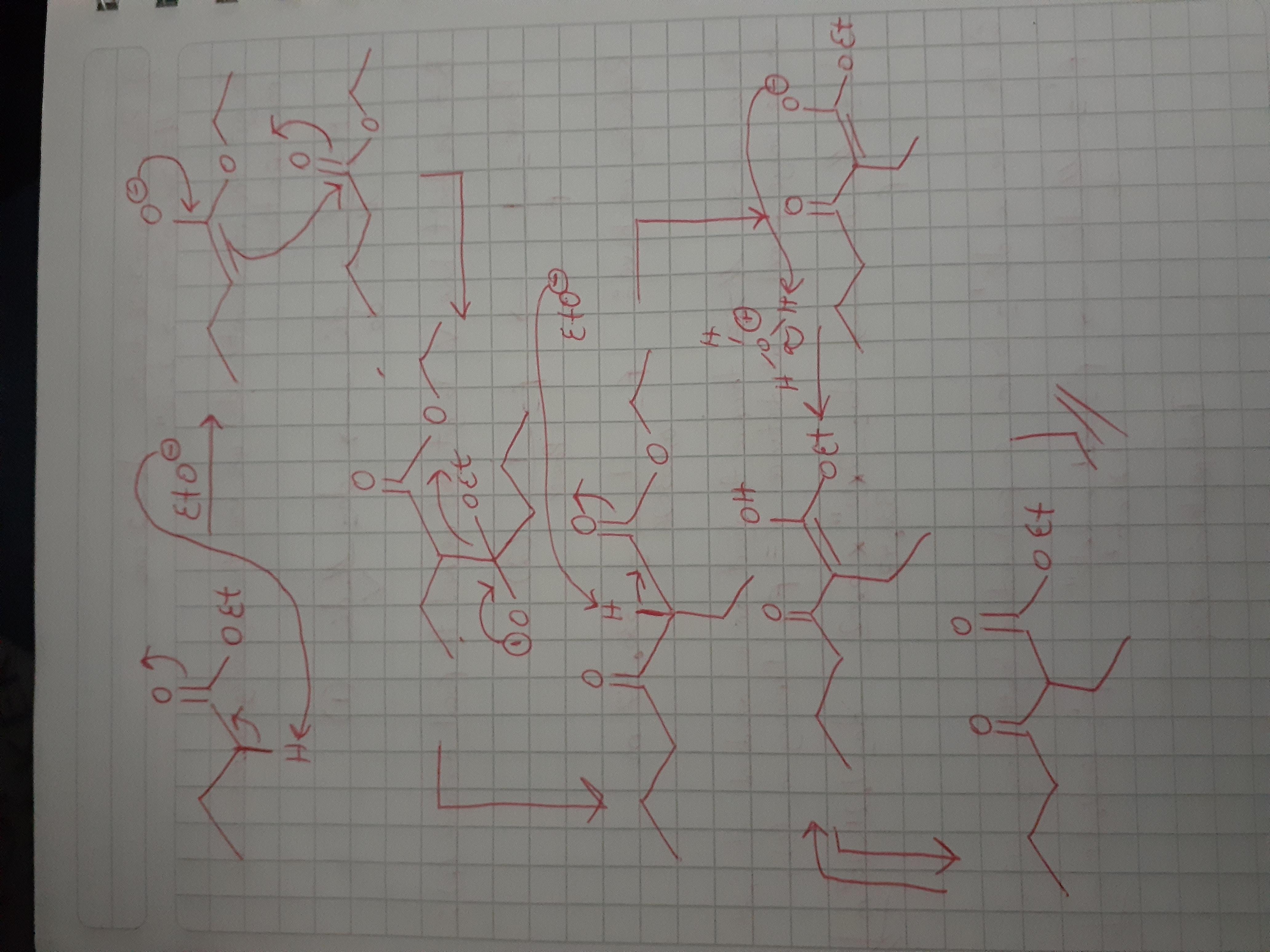
This is a Claisen condensation. In the picture below you have the mechanism and final product.
Hope this helps
Related Questions
Lab 6: Basic Chemistry Thermodynamics: Solve the challenge of storing renewable energy
Answers
Answer:
Expensive and low storing capacity.
Explanation:
The storing of renewable energy is a big challenge for us because this renewable energy can't be stored for a long due to expensive and low storing capacity. There are two challenges about the storing of renewable energy is that the battery are very expensive and can store very less amount of energy which makes unable to store the renewable energy. The scientists should make a storing device which is not too much expensive and has more storage capacity.
If 0.60 L of a solution contains 6.6 g of NaBr, what is its molar concentration?
Answers
Answer:
Molarity = moles ÷ liters
to get moles of NaBr divide grams of NaBr by its molar mass (mass of Na + mass of Bromine)
Na = 22.989769
Br = 79.904
molar mass of NaBr = 102.893769
6.6g ÷ 102.893769 = 0.064143826 moles of NaBr
0.064143826 moles ÷ 0.60 liters = 0.1069 molar concentration or 11 %
How long does it take to fill one train car full of nuclear power can keep a power plant running
Answers
1.6 x 1023 atoms of Be, how many moles of Be are there?
Answers
Answer:
1 mole.
Explanation:
1.6x10^23 = NA = 1 mole of any substance.
Que uso le darías a la vida diaria escribe en español plis
Answers
Explanation:
Facilitar el movimiento y desplazamiento de objetos pesados reduciendo el rose del objeto contra el suelo, Obtener un movimiento circular por efecto de la fuerza de un liquido, en el caso de contadores, molinos, centrales hidroeléctricas y turbinas.
Transmitir el movimiento de un eje a otro como en el caso de las lavadoras, bicicletas y neveras. Reducir drásticamente el esfuerzo necesario para elevar y mover objetos, como en casos de pozos de agua y ascensores Transformar movimientos giratorios en otros movimientos o viceversa. ,
The ionization potential of Be atom is more than expected because it has -
(a) half filled valence p orbitals
(b) fully filled valence s orbitals
(c) both a and b
Answers
Answer:
(b) fully filled valence s orbitals
Explanation:
Electron configuration of Be: 1s22s2
2s2 is fully filled
What is the new concentration? L
M NaCl
Answers
Answer:
Explanation:
\we must convert the mass of NaCl in grams into moles. We do this by dividing by the molecular weight of NaCl (58.4 g/mole). Then, we divide the number of moles by the total solution volume to get concentration. The NaCl solution is a 0.1 M solution.
Answer:
0.125
Explanation:
boom
PLEASE NO BOTS lol
Ethyne or acetylene is also used in cutting torches. The acetylene is combined with pure oxygen producing a flame with a temperature of 6332 °F or 3500 °C. The combustion of acetylene in the presence of excess oxygen yields carbon dioxide and water:
2C2H2 (g) + 5O2 (g) --> 4CO2 (g) + 2H2O (l)
Calculate the value of ΔS° for this reaction.
A. +689.3 J/mol K
B. +432.4 J/mol K
C. -432.4 J/mol K
D. -122.3 J/mol K
Answers
The correct answer is
C. -432.4 J/mol K
How many atoms are in 1.20 moles of copper?
Answers
Answer:
7.23x10^23 atoms
Explanation:
1 mole = 6.023x10^23
1.20 mole = 6.023x10^23 x 1.2
= 7.23x10^23 atoms
The atoms present in 1.20 moles of copper is 7.23 × [tex]10^{23}[/tex] atoms.
What is atom?A chemical element is made up of atoms, which are the basic components of ordinary matter.
Calculation of atoms
Given data:
n = 1.20 moles.
It is known that.
1 mole = 6.023 × [tex]10^{23}[/tex]
So, 1.20 moles = 6.023 × [tex]10^{23}[/tex] × 1.2 = 7.23 × [tex]10^{23}[/tex] atoms.
Therefore, number of atoms in copper will be 7.23 × [tex]10^{23}[/tex] atoms.
To know more about atom click here.
https://brainly.com/question/1566330
#SPJ2
How many moles of KOH are present in 750. mL of a 0.250 M solution?
Answers
Answer:
Answer: Number of moles of KOH present in solution is 3.75.
Explanation:
0.1 moles
There are 0.1 moles of solute in 250 mL of 0.4 M solution.
1 Answer. 1 mol of NaOH
PLEASE HELP!!! CHEMISTRY
Which one would require more energy to overcome because it is the strongest? Hydrogen bonding or van der waals?
Answers
Jenny has a gold bar in a piece of gold foil both objects are made of 100% pure gold watch and closing can guinea make about the two objects
Answers
Answer:
What is the question your asking?
Explanation:
All atoms of the same element have the same properties.
do carbon has ions charge?
Answers
Carbon has an outer shell consisting of 4 valence electrons. ... Thus, a carbon ion can have a charge of anywhere from -4 to +4, depending on if it loses or gains electrons. Although the most common oxidation states of carbon are +4 and +2, carbon is able to make ions with oxidation states of +3, +1, -1, -2, and -3.
How is an exothermic reaction indicated in an equation?
Answers
[tex] \longrightarrow\sf \pmb {Exothermic \: Reaction}[/tex]
[tex] \tt \green {An \: exothermic \: reaction \: is \: indicated \: by \: writing \: “+Heat” \: or \: “+Heat \: energy” \: or \: “+Energy” \: on \: the \: products \: side \: of \: an \: equation.}[/tex]
Answer: Heat is included as one of the products.
Explanation:
(NH4)2Cr2O7 Cr2O3 + N2 + H2O
If 4.7369 moles of H2O are produced, how many moles of (NH4)2Cr2O7 were reacted?
Answers
Answer:
the original substances in any chemical reaction. products. the resulting substances in any....chromium(III) oxide, and water. (NH4)2Cr2O7(s) → N2(g) + Cr2O3(s) + 4H2O(g).
Match the solutions to the descriptions of the freezing points.
a. One mole of the ionic compound Na3PO4 dissolved in 1000 g H2O
b. One mole of the ionic compound CuSO4 dissolved in 1000 g H2O
c. One mole of the nonelectrolyte C6H12O6 dissolved in 1000 g H2O
1. Highest freezing point
2. Intermediate freezing point
3. Lowest freezing point
Answers
Answer:
1c
2b
3a
Explanation:
Step 1: Write the general expression to calculate the freezing point depression
The freezing point depression is a colligative property, that can be calculated using the following expression.
ΔT = i × Kc × b
where,
i: van 't Hoff factor (number of ion particles per formula unit)
Kc: cryoscopic constant (Kc for water: 1.86 °C.Kg/mol)
b: molality (moles of solute per kilogram of solvent)
All the solution have the same Kc and the same b (1 mol/1 kg = 1 m), so ΔTf variation will depend on i
Step 2: Calculate the freezing point of the Na₃PO₄ solution
Na₃PO₄ has 4 ions (3 Na⁺ and 1 PO₄³⁻), so i = 4.
ΔT = i × Kc × b = 4 × 1.86 °C.Kg/mol × 1 mol/kg = 7.44 °C
T = 0°C - 7.44 °C = -7.44 °C
Step 3: Calculate the freezing point of the CuSO₄ solution
CuSO₄ has 2 ions (1 Cu²⁺ and 1 SO₄²⁻), so i = 2.
ΔT = i × Kc × b = 2 × 1.86 °C.Kg/mol × 1 mol/kg = 3.72 °C
T = 0°C - 3.72 °C = -3.72 °C
Step 4: Calculate the freezing point of the C₆H₁₂O₆ solution
C₆H₁₂O₆ is a nonelectrolyte (it doesn't ionize), so i = 1
ΔT = i × Kc × b = 1 × 1.86 °C.Kg/mol × 1 mol/kg = 1.86 °C
T = 0°C - 3.72 °C = -1.86 °C
8. Sulfur has a first ionization energy of 1000 kJ/mol. Photons of what frequency are required to ionize one mole of Sulfur?
Answers
Answer:
the frequency of photons [tex]v = 1.509\times10^{39}Hz[/tex]
Explanation:
Given: first ionization energy of 1000 kJ/mol.
No. of moles of sulfur = 1 mole
[tex]\Delta E_1 = 1000KJ/mol[/tex]
We know that plank's constant
[tex]h = 6.626\times10^{-34} Js[/tex]
Let the frequency of photons be ν
Also we know that ΔE = hν
this implies ν = ΔE/h
[tex]= \frac{10^6J}{6.626\times10^{-34} Js}[/tex]
[tex]v = 1.509\times10^{39}Hz[/tex]
Hence, the frequency of photons [tex]v = 1.509\times10^{39}Hz[/tex]
4.
How many parents take part in binary fission?
Answers
Answer:
one parent
Explanation:
As one parent cell divides it into two daughter cells and so on.
A student is trying to develop questions to help identify an unknown biome. Which of the following questions would provide helpful information to identify an unknown biome? Select ALL that apply. A. Is sunlight an abiotic factor in this biome? B. What is the average temperature of this biome? C. How much precipitation does this biome receive each year? D. What adaptations do plants and animals have to survive in this biome? E. Is the biome located near an ocean? F. Do plants and animals live in this biome? G. Does carbon cycle in this biome?
Answers
Answer:
I think the answer is C. D. And F.
Explanation:
I’m sorry if I’m wrong :(
Answer:
I think the answer is BCD and maybe F
Explanation:
If you have 1.4 grams of silver (Ag), how many moles of silver do you have?
Answers
Answer: There are 0.0129 moles of silver present in 1.4 grams of silver (Ag).
Explanation:
Given: Mass of silver = 1.4 g
Number of moles is the mass of substance divided by its molar mass.
As molar mass of silver is 107.86 g/mol. Therefore, moles of silver are calculated as follows.
[tex]No. of moles = \frac{mass}{molar mass}\\= \frac{1.4 g}{107.86 g/mol}\\= 0.0129 mol[/tex]
Thus, we can conclude that there are 0.0129 moles of silver present in 1.4 grams of silver (Ag).
Jasmine travels a lot and collects rocks wherever she goes. She was examining two rocks from her collection, and she noticed that they are different types of rock. How could energy have played a role in the different rock types forming?
A. Energy changes rock on different continents in different ways. Each continent on Earth has different rock that might form liquid rock or small rock pieces when exposed to energy.
B. Energy caused one rock type to form, but not the other. Rock that forms because of energy is a different type of rock than rock that forms without energy.
C. Energy from different sources leads to different types of rock. Energy inside Earth melts rock into liquid rock, but energy from the sun causes rock to weather into small pieces of rock.
D. Energy causes different types of rock to change in different ways. Energy changes igneous rock into liquid rock and changes sedimentary rock into small pieces of rock.
Answers
Answer:
C
Explanation:
energy from different sources leads to different types of rocks. energy inside the earth(volcanoes) melts rocks into liquid rock(molten larva) but energy from the sun(heat radiation) causes rock to weather(breakdown) into small pieces of rocks
Why is medical technology good for society?
O A. It creates more jobs to do by hand,
B. It makes bilingspitals bigger.
C. It gives patients more illnesses.
D. It helps doctors treat diseases.
Answers
How many molecules of ethanol, C2H5OH, are contained in a 150. gram sample?
1.96 x 1024
46.0
6.02 x 1023
5.1 x 10-25
Answers
Answer:
1.96 × 10²⁴ molecules
Explanation:
Step 1: Given data
Mass of ethanol (m): 150. g
Step 2: Calculate the number of moles (n) corresponding to 150. g of ethanol
The molar mass of ethanol is 46.07 g/mol.
150. g × 1 mol/46.07 g = 3.26 mol
Step 3: Calculate the number of molecules in 3.26 moles of ethanol
To convert moles into molecules, we need Avogadro's number: there are 6.02 × 10²³ molecules in 1 mole of molecules.
3.26 mol × 6.02 × 10²³ molecules/1 mol = 1.96 × 10²⁴ molecules
A solution at 25 degrees Celsius has a pH of 4.48. What is the pOH of this solution?
0.978
3.17
9.52
10.51
Answers
Answer:
The answer is C = 9.52
Explanation:
I took the test
The pOH of a solution that has a pH of 4.48 at 25°C is 9.52. Details about pH can be found below.
What is pH?pH refers to the power of hydrogen in a substance while the pOH refers to the power of hydroxyll ions. The pH of a solution ranges from 0 - 14 indicating the strength of acidity and alkalinity.
The relationship between the pH and pOH in a solution is given as follows:
pH + pOH = 14
According to this question, a solution at 25 degrees celsius has a pH of 4.48. The pOH of the solution is calculated as follows:
4.48 + pOH = 14
pOH = 14 - 4.48
pOH = 9.52
Therefore, the pOH of a solution that has a pH of 4.48 at 25°C is 9.52.
Learn more about pH at: https://brainly.com/question/15289741
Calculate the volume of sulfur dioxide produced when 27.9 mL O2 reacts with carbon disulfide.
Answers
Answer:
18.6 ml
Explanation:
From the balanced equation given:
Volume of oxygen = 27.9 mL
From the equation, we will see that three moles of oxygen react with CS_2 to give two moles of sulfur dioxide (SO_2)
Also, three mL of O_2 yields two mL of SO_2
Now, to calculate the volume of SO_2; we have:
[tex]=27.9 mL \times \dfrac{2 mL \ of SO_2}{3 mL \ O_2}[/tex]
= 18.6 mL of SO_2
Convert 27.7 kilometers to centimeters.
Answers
Answer:
The correct answer is - 2770000 cm.
Explanation:
1 kilometer = 1000 meter
1 meter = 100 centimeter
1 kilometer = 100*1000 cm
1 km = 100000 cm.
then,
27.7 kilometers = 2.77 × 10^6 centimeters
So, 27.7 kilometers = 27.7 × 100000
= 2.77 × 106 or 2770000 centimeters.
how does the study of genetics and Dna
help the study of evolution
Answers
What is the volume of an object if the density is 8g/cm^3 and has a mass of 16g? *
1 point
8mL
128mL
16mL
2mL
Answers
Answer:
I think the answer is 2mL.
Explanation:
i hope it help.
Which is/are used in nuclear reactors to absorb neutrons?
A. heavy nuclei
B. critical mass
C. chain reactions
D. control rods
Answers
Answer:
A. heavy nuclei
Explanation:
They absorb neuyrons in order to hain stability.
By absorbing neutrons, a control rod prevents the neutrons from causing further fissions.
what cause a mass defect?
○A. The mass of a nucleus is larger than it should be.
○B. The mass of a nucleus cannot be accurately measured.
○C. Mass is converted to the energy binding a nucleus together.
○D. Mass is lost when a particle is released in a reaction.
Answers
Answer: Mass is converted to the energy binding a nucleus together.
Explanation: a p e x
Mass is converted to the energy binding a nucleus together cause a mass defect.Hence , Option (C) is correct.
What is Mass defect ?
The actual atomic mass is less than the predicted mass calculated by adding the masses of nucleons.
This additional mass is accounted for by binding energy that is released when a nucleus is formed.
When a nucleus is formed, some of the mass is converted to energy and this results in the mass defect.
Therefore, Mass is converted to the energy binding a nucleus together cause a mass defect. Hence , Option (C) is correct.
Learn more about mass defect here ;
https://brainly.com/question/11624098
#SPJ2