Answers
Answer:
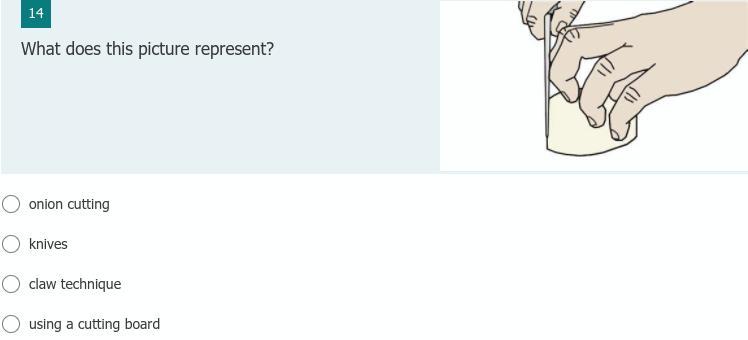
claw techniques
Explanation:
because it is
Related Questions
Birds use their feathers for flight and fluff their feathers to keep warm. Based on Natalia's feather experiment, how do oil spills affect birds’ ability to use their feathers?
Answers
Answer:
As shown previously on Natalia's experiment, oil spills will dangerously affect birds' abilities to user their feathers. When oil gets on a feather, the mass of it increases and it becomes soggy and wet. If birds try to go in water like the ocean, it will not get any better because the ocean is cold. Flying won't be easy because they have additional weight stuck to them.
Explanation:
Hope this helps you!
What is the percent by mass of carbon in carbon dioxide (CO2)?
A. 27%
B. 33%
C. 43%
D. 73%
Answers
If I have 6 moles of a gas at a pressure of 10.6 atm and a volume of 48 liters, what is the temperature of this gas?
An unknown quantity of gas is at a pressure of 14.5 atm, a volume of 45 liters, and a temperature of 97 0C, how many moles of gas exist in this situation?
Answers
Answer: 1). The temperature of this gas is 1032.88 K.
2). There are 21.48 moles of gas exist at a pressure of 14.5 atm, a volume of 45 liters, and a temperature of [tex]97^{o}C[/tex].
Explanation:
1). Given: No. of moles = 6 moles
Pressure = 10.6 atm
Volume = 48 L
Formula used to calculate temperature is as follows.
PV = nRT
where,
P = pressure
V = volume
n = no. of moles
R = gas constant = 0.0821 atm
T = temperature
Substitute the values into above formula as follows.
[tex]PV = nRT\\10.6 atm \times 48 L = 6 mol \times 0.0821 L atm/mol K \times T\\T = \frac{10.6 atm \times 48 L}{6 mol \times 0.0821 L atm/mol K}\\= \frac{508.8}{0.4926} K\\= 1032.88 K[/tex]
Hence, temperature of this gas is 1032.88 K.
2). Given: Pressure = 14.5 atm
Volume = 45 L
Temperature = [tex]97^{o}C = (97 + 273) K = 370 K[/tex]
Formula used to calculate number of moles is as follows.
[tex]PV = nRT\\14.5 atm \times 45 L = n \times 0.0821 L atm/mol K \times 370 K\\n = \frac{14.5 atm \times 45 L}{0.0821 L atm/mol K \times 370 K}\\= \frac{652.5}{30.377} mol\\= 21.48 mol[/tex]
Hence, there are 21.48 moles of gas exist at a pressure of 14.5 atm, a volume of 45 liters, and a temperature of [tex]97^{o}C[/tex].
Organisms that depend on other organisms for food are consumers. Which consumer you think is a herbivore (eats plants only)?
Answers
Answer:
Prairie dog#3
Explanation:
:)
ILL MARK BRAINLIEST :)
Describe the law of conservation of mass in your own words *DO IT IN YOUR OWN WORDS PLEASE*
Answers
Explanation:
According to the law of conservation of mass, mass can neither be created nor destroyed but it can simply be transformed from one form to another.
For example, [tex]Na^{+} + Cl^{-} \rightarrow NaCl[/tex]
Mass of Na = 23 g/mol
Mass of Cl = 35.5 g/mol
Sum of mass of reactants = mass of Na + mass of Cl
= 23 + 35.5 g/mol
= 58.5 g/mol
Mass of product formed is as follows.
Mass of NaCl = mass of Na + mass of Cl
= (23 g/mol + 35.5) g/mol
= 58.5 g/mol
As mass reacted is equal to the amount of mass formed. This shows that mass is conserved.
As a result, law of conservation of mass is obeyed.
How many moles are in 2.8x10^24 atoms of silicon?
Answers
Answer:
4.6 mol Si
General Formulas and Concepts:
Atomic Structure
Reading a Periodic TableMolesAvogadro's Number - 6.022 × 10²³ atoms, molecules, formula units, etc.Stoichiometry
Using Dimensional AnalysisExplanation:
Step 1: Define
[Given] 2.8 × 10²⁴ atoms Si
[Solve] moles Si
Step 2: Identify Conversions
Avogadro's Number
Step 3: Convert
[DA] Set up: [tex]\displaystyle 2.8 \cdot 10^{24} \ atoms \ Si(\frac{1 \ mol \ Si}{6.022 \cdot 10^{23} \ atoms \ Si})[/tex][DA] Divide [Cancel like units]: [tex]\displaystyle 4.64962 \ mol \ Si[/tex]Step 4: Check
Follow sig fig rules and round. We are given 2 sig figs.
4.64962 mol Si ≈ 4.6 mol Si
True or False?
When it is summer in the Northern Hemisphere, it is summer all around the word
Answers
how many carbon atoms are in 26-hydrogen alkyne
Answers
20. Which of the following is the correct name for N205?
O nitrate oxide
O dinitrogen pentoxide
O nitrous oxide
O nitrogen dioxide
Answers
Answer:
B!
Explanation:
Di stnds for 2 (N2)
pent = 5 (05)
If the half-life of a radioactive substance is 500 million years and you have 48 atoms of it, how many half-lives will have passed by the time that 6 atoms of the substance remain?
O A. 3
O B. 2
O C. 4
O D. 5
Answers
Answer: 3
Explanation:
What do plants do with sugar? *
Release it through their stoma
Eat it for energy
Convert it into oxigen
Convert it into water
Answers
Answer:
its B .Eat it for energy.
what does climate change have to do with glacial change
Answers
Answer:
Because glaciers are so sensitive to temperature fluctuations accompanying climate change. so if it gets hotter then the ice glaciers will melt.
Explanation:
how many layer does the earth have?
Answers
Answer:
The earth is split into four major layers: the crust, the mantle, the outer core and the inner core
Explanation:
Write the formulas for the following compounds :
Sodium phosphate
Phosphorus triiodide
Answers
phosphorus triiodide = PI3
hope this helps. make brainliest please!
Explanation:
1.Na3PO4
2.PI3
therefore this is the formula for sodium phosphate and phosphrous triiodide
How many pairs of electrons are shared by adjacent carbon atoms in an alkane?
Answers
Answer:
one
Explanation:
What is the hydroxide ion concentration of a solution with a pH of 4?
A. 1x10^-4
B. 1x10^-7
C. 1x10^-14
D. 1x10^-10
Answers
Answer:
Jules
Explanation:
If 2.28 g C8H18 reacts with 7.00 g of Oz, predict the mass of CO, that can be produced and
identify the limiting reactant. Explain how you got your answers.
Unbalanced equation: C8H180 + 026) • CO26) + H₂O
Answers
Answer:
O₂ is limiting reactant and 6.17g CO₂ can be produced
Explanation:
Based on the balanced reaction:
C₈H₁₈ + 25/2O₂ → 8CO₂ + 9H₂O
1 mole of C₈H₁₈ reacts with 25/2 moles O₂.
To solve this question we must convert the mass of each reactant to moles and, using the balanced reaction, we can find limiting reactant. With moles of limiting reactant we can find the moles of CO₂ and its mass as follows:
Moles C₈H₁₈ -Molar mass: 114.23g/mol-
2.28g * (1mol / 114.23g) = 0.0200 moles C₈H₁₈
Moles O₂ -Molar mass: 32g/mol-
7.00g * (1mol / 32g) = 0.219 moles O₂
For a complete reaction of 0.0200 moles C₈H₁₈ are needed:
0.0200 moles C₈H₁₈ * (25/2moles O₂ / 1mole C₈H₁₈) = 0.250 moles of O₂ are needed.
As there are just 0.219 moles, O₂ is limiting reactant.
Moles of CO₂ that can be produced:
0.219 moles O₂ * (8moles CO₂ / 25/2moles O₂) = 0.140 moles CO₂
The mass is -Molar mass CO₂: 44.01g/mol-:
0.140 moles CO₂ * (44.01g / mol) =
6.17g CO₂ can be producedA heterogeneous mixture always contains
only one substance.
O more than two substances.
O two or more substances that are visibly distinguishable.
O two or more substances that are not visibly distinguishable.
Answers
Answer:
two or more substances that are visibly distinguishable.
How many electrons does GeH4 have
Answers
Ge has 32 electrons. H has 1 electron.
32 + 1(4) = 36
what is chemical bonding?
Answers
Answer:
Chemical bonding - a mutual adhesion of atoms in an molecule and the crystal lattice.
Explanation:
Hope you have a great day
True or False: Molecular formula gives you the smallest whole number ratio of elements in a compound. *
Answers
Answer:true
Explanation:The molecular formula indicates the exact number of atoms in the molecule. The empirical formula expresses the smallest whole number ratio of the atoms in the element.
On the left a Circuit A, which is a square box. The top, left, and right sides have circles with X's in them. The bottom side has a stack of vertical lines, which are from left to right very short, short, very short, short. On the right a Circuit B, which is a square box. 2 extra lines cross the box near its top, parallel with the top side. The top side and the 2 extra lines have circles with X's in them. The bottom side has a stack of vertical lines, which are from left to right very short, short, very short, short.
Use the diagram and drop-down menus to answer each question.
Which circuit is a parallel circuit?
Which circuit is a series circuit?
In which circuit do the light bulbs all shine at their maximum brightness?
Answers
Answer:
B A B sorry if its wrong
Explanation:
Circuits can be connected in series or parallel but the maximum voltage is obtained in series connection, thus, light bulbs shine brightest in series connection.
What is parallel and series connection in a circuit?A parallel circuit connection is one in which the terminals of all the components in th circuit meet at two points.
The components are parallel to each other and current flows in multiple directions.
A series connection in a circuit is one in which the terminals of the component parts in a circuit are joined end to end. Current flows in one direction.
The voltage in a series connection is the sum of all the individual cells, therefore, the light bulb in series connection shines brightest.
Learn more about parallel and series circuits at: https://brainly.com/question/1122566
#SPJ2
The best way to increase the amount of a solid solute dissolved in a saturated solution would be to
Answers
Answer:
The solubility of a saturated solution can be increased by increasing the temperature.
Explanation:
Temperature -- Generally, an increase in the temperature of the solution increases the solubility of a solid solute. For example, a greater amount of sugar will dissolve in warm water than in cold water. A few solid solutes, however, are less soluble in warmer solutions.
How is entropy related to the spontaneity of a reaction? AP3X
Answers
Answer:
S>0 contributes to spontaneity.
Explanation:
i just took the test on a pex :)
According to the law of thermodynamics, the relationship between entropy and the spontaneity of a reaction is S > 0.
What is entropy?Entropy is the total amount of energy in a closed system that is present there, but there are no uses for that energy. It is the unused energy for any work.
By the second law of thermodynamics, any spontaneous process results in an increase in the universe's overall entropy. The total of the entropy produced by the spontaneous process and the change in energy brought on by the heat flow is known as the net change in entropy of the system or S.
The reaction will be spontaneous if the delta G is negative. Delta G is the Gibbs free energy. So the entropy S will be greater that the reaction.
The Gibbs free energy equation is:
△G = △H − T△S
Thus, the relation is S > 0.
To learn more about entropy, refer to the link:
https://brainly.com/question/13386678
#SPJ2
How do textbooks usually describe Le Châtelier's famous principle
Answers
Answer:is an observation about chemical equilibria of reactions. It states that changes in the temperature, pressure, volume, or concentration of a system will result in predictable and opposing changes in the system
Explanation:
If a book has a weight of 23.2 N on Earth, what is its mass?
Answers
Answer: Mass is 2,37 kg
Explanation: Weight G = mg, and g = 9.81 m/s² on Earth.
m = W/g = 23.2 N / 9.81 m/s²
When you mix water and salt you get a.
Answers
How many grams of copper (II) oxide will react with 10 liters of hydrogen gas?
Answers
Answer:
maybe 200g
I don't know thats just a guess
HOPE ITS TURE
A car engine produces 604 kJ of mechanical energy from fuel capable of producing 2416 kJ of energy. The efficiency of the engine is
Answers
A car engine produces 604 kJ of mechanical energy from fuel capable of producing 2416 kJ of energy, then efficiency of the engine is 25%.
How do we calculate efficiency?We can calculate the efficiency by using the below equation as:
Efficiency = (Actual production / Theoretical production) × 100%
Given that,
Actual production = 604 kJ
Theoretical production = 2,416 kJ
On putting values in given equation, we get
Efficiency = (604/2,416) × 100% = 25%
Hence efficiency of the engine is 25%.
To know more about efficiency, visit the below link:
https://brainly.com/question/15418098
#SPJ2
Is this correct??Pls help!!!
Answers
Answer:
C
Explanation:
Answer:
Yes, your answer is correct. B is correct :3