HELP ASAP 10 POINTSSS
In this diagram, the medium on top is air and the medium on bottom is glass. Which vocab word best describes what is happening when the light wave passes from the air into the glass?
Question 2 options:
resolution
diffraction
refraction
reflection
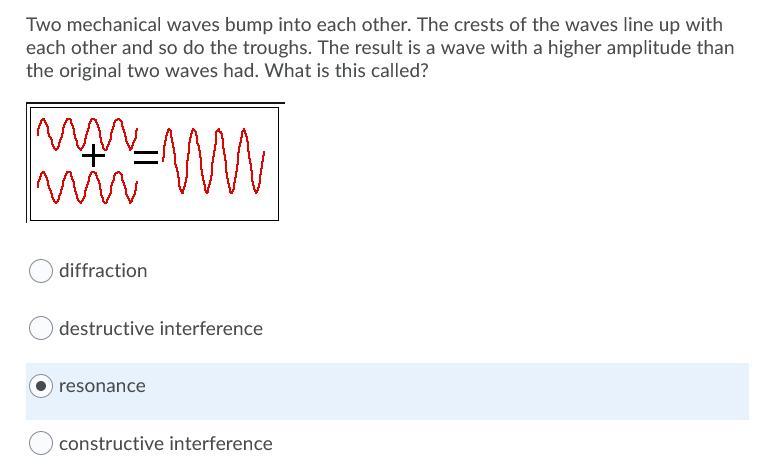
Answers
Answer: refraction
Explanation: this is because during refraction light moves from one medium to another.
Related Questions
5. How would you calculate the pH (NOT POH) of a base when given
the concentration of [OHJ?
Answers
Answer:
pOH = -log₁₀ [OH-]
pH = 14 - pOH
Explanation:
First of all, calculate the value of pOH from the hydroxide concentration do this using the equation below;
pOH = -log₁₀ [OH-]
After obtaining the pOH, calculate pH using the equation below:
pH + pOH = 14
pH = 14 - pOH
A salt is not composed of:
A. a metallic cation
B. non-metallic anion
C. an anion of a base
D. an anion of an acid
Answers
Answer:
B: non metalic anion if you try chemistry up salt is non metalic with metal things...
Explanation:
Does this help?
At STP, which of the following would have the same number of molecules at 1 L of C2H4 gas? Assume that each behaves as an ideal gas.
A. 0.5 L of H2 gas
B. 2 L of H2O gas
C. 1 L of Ne gas
D. 3 L of Cl2 gas
Answers
Answer: 1L of Ne gas
The answer is Option C , 1L of Ne gas will have the same number of molecules at 1 L of C₂H₄ gas
What is STP ?At standard temperature and pressure, a system is said to have a temperature of zero degrees centigrade (273 Kelvins) and the pressure equal to the atmosphere is always 1 atm.
Additionally, one mole of any gas at STP occupies a volume of 22.414 L.
This concept only holds true for gases.
So for a gas having same pressure , temperature and moles according to the Ideal Gas Law , volume also needs to be same
pV=nRT
and in the given option ,Option C has the same volume as that of 1 L of C₂H₄ gas .
To know more about STP
https://brainly.com/question/26107741
#SPJ2
Describe and show an example of valence electrons. What is a common trend you can remember to help you understand valence electrons?
Answers
Explanation:
valency from right to left in a Periodic Table in same period first increases then decreases and from top to bottom it is same for a same group
1. If you have 8.1 g CO₂ and 2.3 g H₂, which one is the limiting reactant. Show your work. 2. Calculate the theoretical yield of CH₄.
Answers
Answer:
2.95 g of CH₄
Explanation:
To start this, we determine the equation:
4H₂ + CO₂ → CH₄ + 2H₂O
4 moles of hydrogen react to 1 mol of carbon dioxide in order to produce 1 mol of methane and 2 moles of water.
To determine the limiting reactant, we need to know the moles of each reactant.
8.1 g . 1 mol/ 44g = 0.184 moles of carbon dioxide
2.3 g . 1mol / 2g = 1.15 moles of hydrogen
4 moles of hydrogen react to 1 mol of CO₂
Then, 1.15 moles may react to (1.15 . 1) /4 = 0.2875 moles
We only have 0.184 moles of CO₂, so this is the limiting reactant. Not enough CO₂ to complete the 0.2875 moles that are needed.
Ratio is 1:1. 1 mol of CO₂ produces 1 mol of methane
Then, 0.184 moles of CO₂ will produce 0.184 moles of CH₄
We convert moles to mass: 0.184 mol . 16 g /mol = 2.95 g
what do electrons in the same shell have in common
Answers
Answer:
They have the same amount of energy. They are all positively charged. They are all made up of atoms.
Answer:
they have the same quantity of energy...
What mass of potassium would need 2350 J of energy in order to raise its temperature from 44.3°C to 57.8°C? (cpotassium = 0.753 J/g°C)
Answers
Answer:
Explanation:
q = mCΔT
m =CΔT/q
ΔT = 57.8°C - 44.3°C = 13.5°C
m = (0.753*13.5)/2350
m = 0.00433 g
The mass of potassium that will need 2350 J of energy in order to raise its temperature from 44.3°C to 57.8°C is 231.17 g
Data obtained from the question Heat (Q) = 2350 JInitial temperature (T₁) = 44.3 °CFinal temperature of water (T₂) = 57.8 °CChange in temperature (ΔT) = 57.8 – 44.3 = 13.5 °C Specific heat capacity (C) = 0.753 J/gºC Mass (M) =?Q = MCΔT
2350 = M × 0.753 × 13.5
2350 = M × 10.1655
Divide both side by 10.1655
M = 2350 / 10.1655
M = 231.17 g
Learn more about heat transfer:
https://brainly.com/question/6363778
#SPJ2
What change would shift the equilibrium system to the left?
A(g) + B(s) + Energy = 309) (3 points)
Adding more of gas C to the system
Heating the system
Increasing the volume
Removing some of gas C from the system
Answers
Answer:
C
Explanation:
sorry if its wrong but i think its right
Heating the system will shift the equilibrium system to the left.
The correct option is B.
What is equilibrium?
Chemical equilibrium is a state where no net change in the amounts of reactants and the products occurs during a reversible chemical reaction.
A reversible chemical reaction happens when the products react with the original reactants as soon as they are generated.
On a treadmill, you are walking continuously but not going anywhere, which can be said as equilibrium.
Thus, the correct option is B.
Learn more about equilibrium
https://brainly.com/question/13458865
Help meeeeee
A 280.0 sample of neon gas exerts a pressure of 660.0 mm Hg at 26.0°C. Assuming that the pressure remains constant, at what temperature in would the volume change to 440.0 ml?
Answers
Answer:
469.9K
Explanation:
Using Charles's law equation:
V1/T1 = V2/T2
Where;
V1 = initial volume (ml)
V2 = final volume (ml)
T1 = initial temperature (K)
T2 = final temperature (K)
Based on the information provided:
V1 = 280.0ml
V2 = 440.0 ml
T1 = 26.0°C = 26 + 273 = 299K
T2 = ?
Using V1/T1 = V2/T2
280/299 = 440/T2
0.936 = 440/T2
T2 = 440 ÷ 0.936
T2 = 469.9K
Find the total pressure (in atm) of a mixture that contains 3 gases with the following partial pressures. Gas A is 4.2atm, Gas B is 780 mmHg, Gas C is 250.00kPa
Answers
Answer: The total pressure of given mixture that contains 3 gases is 7.68 atm.
Explanation:
Given: Partial pressure of gases is:
Gas A = 4.2 atm
Gas B = 780 mm Hg
Convert mm Hg into atm is as follows.
[tex]1 mm Hg = 0.00131579 atm\\780 mm Hg = 780 mm Hg \times \frac{0.00131579 atm}{1 mm Hg}\\= 1.02 atm[/tex]
Gas C = 250 kPa
Convert kPa into atm as follows.
[tex]1 kPa = 0.00986923 atm\\250 kPa = 250 kPa \times \frac{0.00986923 atm}{1 kPa}\\= 2.46 atm[/tex]
Total pressure is the sum of partial pressures of gases present in the mixture.
Therefore, total pressure of given mixture is calculated as follows.
[tex]P_{total} = P_{A} + P_{B} + P_{C}\\= 4.2 atm + 1.02 atm + 2.46 atm\\= 7.68 atm[/tex]
Thus, we can conclude that the total pressure of given mixture that contains 3 gases is 7.68 atm.
How many protons, neutrons, and electrons are found in a neutral atom of hydrogen-333?
Answers
Answer:
Explanation:
A hydrogen atom has an atomic number of 1.
So it's proton number will always be 1.
A neutral hydrogen atom does not have any charge.
So it has the same amount of electrons as protons, which turns out to be also 1.
A neutral hydrogen atom has an atomic mass of ≈
1.00794 amu ≈ 1 amu.
To find the amount of neutrons, we take the proton number and subtract it from the mass number.
1 − 1 = 0
So a neutral hydrogen atom has no neutrons.
In summary,
A hydrogen atom has
1 proton
1 electron
0 neutrons
I hope that helps!
What is a jet stream. in your own words but be more detailed about what you say.
(don't send a link)
Answers
5 difference between Ionic compound and covalent compound
Answers
Answer:
Ionic compounds are formed by the transfer of electrons that are positively and negatively charged, whereas, covalent compounds are formed by sharing the electrons. 2. In an ionic compound, bonding involves a metal and nonmetal, whereas, in the covalent compound, bonding is between nonmetals
what is electromagnetic?
Answers
Answer:
relating to the interrelation of electric currents or fields and magnetic fields.
2 Iron(III) oxide (Fe2O3) is reduced by carbon on heating to give iron metal (Fe) and
carbon dioxide (CO2). (Ar values Fe 56, C 12,0 16)
When 480 g of Fe2O3 is heated with carbon, 336 g of Fe and 198 g of CO2 are produced.
Use the Law of Conservation of Mass to work out the mass of carbon that
reacted.
a
no links please i need this ASAP
Answers
Using the law of conservation of mass, the mass of carbon reacted is 54 g of carbon.
What is the law of conservation of mass?According to the law of conservation of mass, matter is neither created nor destroyed. This means that the total mass of reactants is equal to the total mass of products.
Total mass of the products = 336g + 198g = 534 g
Total mass of reactants = 480 g + x g
From the law of conservation of mass; 534 g = 480 g + x g
x g = 534 g - 480 g
x g = 54 g
Hence, 54 g of carbon reacted.
Learn more about law of conservation of mass: https://brainly.com/question/13383562
147.0 g piece of metal was heated to 155 C and added to a calorimeter containing 75.0 grams of water. The temperature of the water in the calorimeter was 23.5 C. After the metal was added, the temperature rose to 33.5 C. What is the specific heat of the metal?
Answers
4. At what temperature will 5.00 g of Cl, exert a pressure of 900. torr at a volume of 750 ml?
Answers
Answer:
p=27.8atm
Explanation:
P = 27.8atm
At what temperature will 5.00 g of Cl2 exert a pressure of 900. mmHg at a volume of 750.
What will the volume be of 50 moles of helium at 25°C and 2.5 atm?
Answers
Which of the following is not a fossil fuel?
o copper
natural gas
O petroleum
O coal
Answers
Answer:
copper
Explanation:
coal, petroleum, and natural gas are all related to fossil fuels.
Copper is not a fossil fuel and the correct option is option 1.
What are fossil fuels?Fossil fuels are compound mixtures made of fossilized plant and animal remnants from millions of years ago. The creation of fossil fuels—either oil, natural gas, or coal—from these fossils is determined by the type of fossil, the amount of heat, and the amount of pressure.
Fossil fuels are a non-renewable source of energy. Most of the energy used by us is obtained by the burning of fossil fuels. These fossil fuels are used up at a faster rate. They cannot be regrown at a scale compared to their consumption.
Therefore, Copper is not a fossil fuel and the correct option is option 1.
Learn more about Fossil fuels, here:
https://brainly.com/question/3371055
#SPJ2
A balloon contains 25.0 L of helium gas at 177 kPa. What is the volume when the balloon rises to an altitude where the pressure is only 43.0 kPa ? Assume the temperature remains constant.
A.34.2 L
B.68.8 L
C.46.5 L
D.103 L
Answers
Describe the three-dimensional property of an electromagnetic wave. Use the diagram below
Answers
Answer: The are waves that consist of vibrating electric and magnetic fields. They transmit energy through matter or across space. Some electromagnetic waves are generally harmless. Like other waves, electromagnetic waves have properties of speed, wavelength, and frequency.
Explanation:
Consider the reaction.
2HF(g) +
H2(g) + F2(g)
At equilibrium at 600 K, the concentrations are as follows.
[HF] = 5.82 x 10-2 M
[H2] = 8.4 x 10-3M
[F2] = 8.4 x 10-3M
What is the value of Kea for the reaction expressed in scientific notation?
O 2.1 x 10-2
102.1 x 102
1.2 x 103
0 1.2 x 10-3
Answers
Answer:
0.021
Explanation:
Step 1: Write the balanced equation
2 HF(g) ⇄ H₂(g) + F₂(g)
Step 2: Calculate the value of the concentration equilibrium constant
The concentration equilibrium constant (K) is equal to the product of the concentrations of the products raised to their stoichiometric coefficients divided by the product of the concentrations of the reactants raised to their stoichiometric coefficients.
K for this reaction is:
K = [H₂] × [F₂] / [HF]²
K = (8.4 × 10⁻³) × (8.4 × 10⁻³) / (5.82 × 10⁻²)² = 0.021
Help i’m taking a test!!
What new element is formed from the equation below? (Answer form is
MN:, AN: , S)
Answers
Answer:
Element Z
Explanation:
I hope this is correct and helps you!
Would an alkali metal make a good replacement for tin in a tin can? Explain.
Answers
Answer:
No
Explanation:
If tin is heated, it can react with alkalis' with the release of hydrogen.
Tin is a great metal that is used for packaging purposes.
It does not react with any acid that may be present in the food material stored inside the tin can which makes it a useful metal. It keeps the food safe.However, if the tin in the tin can is replaced with alkali metals then they will readily react with the acid present in the food materials stored inside, thereby rendering the food unsafe.
https://brainly.com/question/18153051
How many moles of O₂ are needed to react completely with 35.0 mol of FeCl₃?
4FeCl3+3O2->2Fe2O3+3Cl2
The 2 for the second half of the equation is not a coefficient it’s supposed to be for 2Fe2 Not 2O3.
26.3 mol
46.7 mol
23.3 mol
10.0 mol
Answers
Answer:
26.3 mol O₂
Explanation:
Step 1: Write the balanced equation
4 FeCl₃ + 3 O₂ ⇒ 2 Fe₂O₃ + 6 Cl₂
Step 2: Establish the appropriate molar ratio
According to the balanced equation, the molar ratio of FeCl₃ to O₂ is 4:3.
Step 3: Calculate how many moles of O₂ are needed to react completely with 35.0 mol of FeCl₃
We will use the previously established molar ratio.
35.0 mol FeCl₃ × 3 mol O₂/4 mol FeCl₃ = 26.3 mol O₂
Titaium oxside is often added to food to color it white. If a jelly bean contains approximately 1.28x10-5 moles of TiO2, how many grams of TiO2 are in a jelly bean
Answers
Answer:
1.02 × 10⁻³ g
Explanation:
Step 1: Given data
Number of moles of titanium (IV) oxide in 1 jelly bean (n): 1.28 × 10⁻⁵ moles
Step 2: Calculate the mass (in grams) corresponding to 1.28 × 10⁻⁵ moles of TiO₂
To convert moles to mass, we need a conversion factor. In this case, it is the molar mass of TiO₂: 79.87 g/mol.
1.28 × 10⁻⁵ mol TiO₂ × 79.87 g TiO₂/1 mol TiO₂ = 1.02 × 10⁻³ g TiO₂
Helpp me please answer fast
Answers
Answer: An element is a substance only containing atoms that have the same number of protons in the chemical nuclei
Explanation:
Type the correct answer in each box.
Answers
Answer:
1 . 26
2. 55.854
Explanation:
because atomic number is the number of proton in other words smaller number and the mass number is the number of neutrons and electrons in other words bigger number .
hope this make sense :)
Identify two ways that temperature plays a role in chemical changes.
Answers
Answer:
Increasing the temperature will cause chemical changes to occur faster. Decreasing the temperature, causes the particles to lose energy which causes them to move around less and slower. The less they move, the less collisions occur, and the less reactions occur between the chemicals = slower reaction rate.
Explanation:
which process is a chemical change